Prevent Cancer Foundation supports FDA rule to ensure patients are informed about breast density
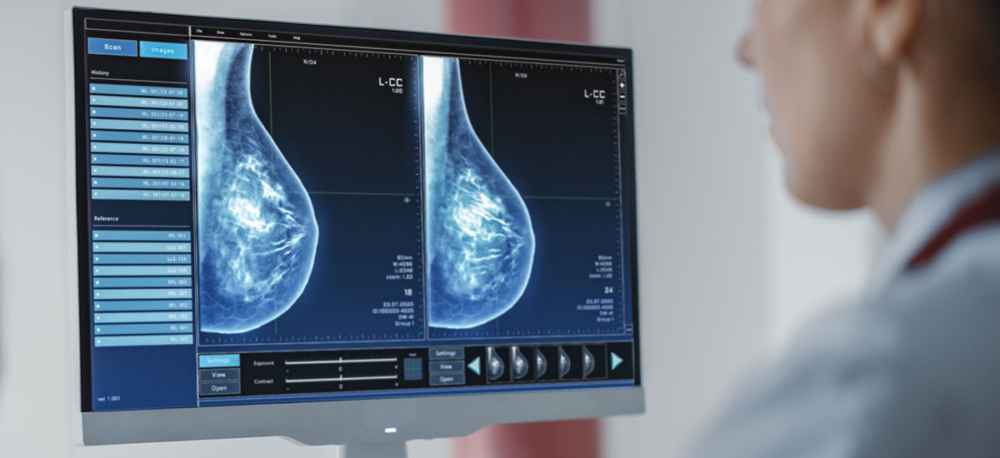
Greater breast density is linked with a higher risk for breast cancer. But without having that information readily available after a mammogram, how can one determine whether they have dense breasts—and what proper steps to take if they do?
As of Tuesday, September 10, the U.S. Food and Drug Administration (FDA) will require all mammography facilities to include a breast density assessment as part of a patient’s medical report. All summaries sent to patients following their mammogram will be required to say whether their breasts are “dense” or “not dense.” Prior to this rule, some states had already required screening facilities to notify patients of their breast density, but thanks to updated FDA regulations it is now a national requirement.
Breast density cannot be determined by how breasts feel or look, but it can be seen on a mammogram, which is an x-ray of the breast. Both 2D and 3D (tomosynthesis) mammograms show dense areas of the breast as white, while areas that are less dense (or more fatty) appear dark.
In addition to increasing overall breast cancer risk, dense breast tissue can make it harder for the radiologist reading the mammogram to see breast cancer. This is because breast cancer most often appears white on a mammogram. If breast cancer occurs in an area of dense breast tissue, which also appears white on a mammogram, the radiologist may miss it. This underscores the importance of patients understanding their breast density and options for additional imaging.
Having dense breasts is completely normal and is quite common—almost half of all women ages 40 and over have dense breasts. Prior to the FDA’s notification requirement, which standardizes language on this issue, breast density reporting could be confusing to decipher and didn’t always contain information about next steps following a dense breasts notification. While these changes based on FDA requirements are helpful, more could be done to provide better education on what breast density is and how it impacts health. If someone is notified that they have dense breasts, they should talk to their health care provider to discuss potential next steps and develop a personalized plan to achieve better outcomes for their health.
“We applaud the FDA on this important rule to increase patient knowledge, but supporting a patient through their breast density assessment is a crucial component to achieving better health outcomes,” said Jody Hoyos, CEO of the Prevent Cancer Foundation. “Continued education around breast density—a known risk factor for breast cancer—is necessary to ensure everyone feels empowered to discuss their results with a provider.”
Depending on a person’s breast density and personal risk of breast cancer, next steps may include supplemental breast magnetic resonance imaging (MRI) or breast ultrasound, to help confirm the mammogram reading. Unfortunately, follow-up imaging may not be covered by insurance—posing a significant barrier to those who need it. The Prevent Cancer Foundation and other national advocacy organizations support federal legislation which, if passed, would remove financial and administrative barriers to necessary breast imaging, ensuring all people can follow recommended screening guidelines from their providers based on breast density and individual risk.
Breast cancer is an issue of health equity, particularly for Black women, who, despite being less likely to be diagnosed with breast cancer, are often diagnosed with more advanced stage disease and are more likely to experience lower quality screening. These disparities reveal the need for better screening methods and standards to ensure high-quality results and comprehensive follow-up care across all populations.
“We must make better outcomes accessible for everyone. The Prevent Cancer Foundation will continue to advocate for legislation that enables everyone to receive the necessary follow-up care they need to detect cancer early,” said Ms. Hoyos.
Regardless of signs or symptoms, those who were assigned female at birth and have breasts should begin annual screening mammography at age 40 if they are of average risk. Transgender individuals should talk with their health care provider about their specific screening needs.
Early Detection = Better Outcomes. For more information about breast cancer, visit preventcancer.org/breast.